Background information regarding Immunofluorescence:
- Indication: Direct and indirect immunofluorescence plays a role in the evaluation of immunobullous diseases and their mimics, and in the investigation of vascular injury syndromes and collagen vascular disease.
- The two principle techniques employed in immunofluorescent (IF) testing are direct and indirect IF.
- Direct immunofluorescence (DIF) testing employs fluorescein-conjugated antibodies monospecific for immunoglobulin (Ig) G, IgM, and IgA and complement fractions directly overlaid on frozen sections of patient tissue.
- Indirect immunofluorescence (IIF) by definition employs a linking antibody, and that term should be used irrespective of the test substrate, be it patient lesional tissue or non-human esophagus, skin or bladder. However, in common terminological usage, IIF is used to refer to the application of patient serum in concert with fluorescein-conjugated anti-IgG and a mucosal substrate such as guinea pig or monkey esophagus or rat bladder.
- The 3 main disease categories in which DIF may be a critical diagnostic adjunct are vesiculobullous disorders, vasculitis, and autoimmune connective tissue disease
Specimen requirements:
Direct IF testing:
- 3 or 4 millimeter punch biopsy placed in physiologic fixative. The specimen should be shipped overnight.
Immunoreactants panel:
- IgG, IgM, IgA, C3, C1q, C3d, C4d, and C5b-9.
- Additional studies offered: Salt split skin studies to discriminate between bullous pemphigoid and epidermolysis bullosa acquisita; the Alport's panel to rule or out Alport's syndrome, specifically assessing for the presence or absence of the alpha 5 chain of type IV collagen in skin, the most common genetic mutation implicated in Alport's syndrome.
Indirect IF testing:
- Sample requirement: Blood placed in a serum separator tube
- Method of transport: Specimen is shipped overnight at room temperature
- Tests offered: assess for circulating pemphigus and pemphigoid associated antibodies. Assess for circulating anti-endothelial cell antibodies characteristic of dermatomyositis, systemic scleroderma and Susac's syndrome.
Results:
The biopsy is interpreted within 24 hours of receipt of the specimen.- All specimens are interpreted by Dr. Magro. She is available 24/7 and can be reached via cell phone.
Examples of Immunofluorescent studies performed at Weill Cornell Medicine:
The Salt Split Skin Assay:
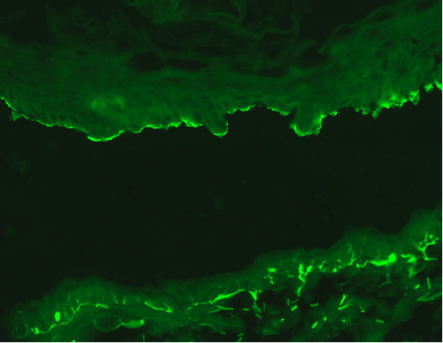
The salt split skin assay shows localization of immunoreactivity to the roof of the saline induced split typical for bullous pemphigoid.
Specimen provided must not have an in vivo split. The salt split skin separation is performed in vitro.

Direct immunofluorescent studies will show homogeneous linear staining along the dermal-epidermal junction. Salt split skin studies show localization to the floor of the saline induced split characteristic of anti-epiligrin pemphigoid (Magro CM and Wu R et al Int J Dermatol 2012).
The C5b-9 Assay
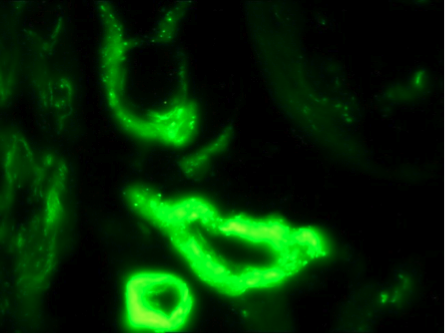
Bullosa Diabeticorum
In both porphyria and diabetes mellitus, a striking pattern of C5b-9 deposition in vessels is observed. The staining pattern is both granular and homogeneous in nature (Vasil K and Magro CM, J Am Acad of Dermatol 2007).Dermatomyositis
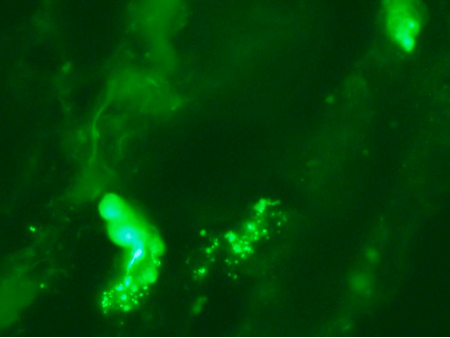
C5b-9 deposition in vessels is a cardinal hallmark in the setting of dermatomyositis (Crowson and Magro Human Pathol 1996)
The Anti-Endothelial Cell Antibody Assay
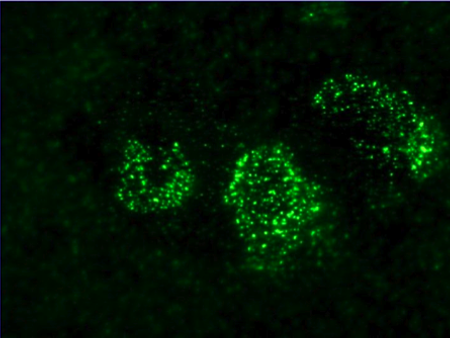
The antiendothelial antibody assay is very useful in certain clinical settings. In this particular photomicrograph the patient's serum has been incubated with generic endothelial cells. There is a very extensive positive granular nuclear staining pattern. The patient's have a positive antiendothelial cell antibody assay. This patient had underlying dermatomyositis. Dermatomyositis is among the prototypic immune based microvascular injury syndromes. (Magro CM et al. Am J Clin Pathol 2007)
Disorders with a Positive Immunofluroescent Profile
Autoimmune vesiculobullous disorders:- Antibodies directed to components of the epidermal basement membrane zone: Bullous and cicatricial Pemphigoid
- Herpes Gestationis
- P200 pemphigoid
- Linear IgA disease
- Bullous systemic lupus erythematosus
- Epidermolysis bullosa acquisita
- Pemphigus foliaceus
- Pemphigus vulgaris
- IgA pemphigus
- Drug induced pemphigus
- Paraneoplastic pemphigus
- Dermatitis Herpetiformis
- IgA vasculitis
- Mixed cryoglobulinemic vasculitis
- Urticarial vasculitis
- Lupus erythematosus (specifically in the context of the positive lupus band test)
- Lupus erythematosus associated with anti-Ro antibodies
- Dermatomyositis
- Antiphospholipid antibody syndrome
- Degos Disease
- Cocaine associated retiform purpura
- Subset of patients with thrombotic thrombocytopenic purpura
- Porphyria
- Pseudoporphyria
- Bullosa diabeticorum
- Lupus erythematosus
- Mixed connective tissue disease
- Dermatomyositis
- Sclerodermatomyositis
Vasculopathic disorders with distinctive Immunofluorescent Profiles
- IgA vasculitis/Henoch Schonlein Purpura
- Mixed cyroglobulinemic vasculitis
- ANCA positive vasculitic syndromes
- Hypocomplementemic urticarial vasculitis
- Antiphospholipid antibody syndrome
- Cocaine induced retiform purpura
Dr. Cynthia Magro, Director of Dermatopathology at Weill Cornell Medical College and Professor of Pathology and Laboratory Medicine, is one of the leading experts in the country in the interpretation of immunofluorescent specimens. She has authored over 250 papers in the field of Dermatopathology. Many of her papers focus on the application of direct and indirect immunofluorescent studies in the evaluation of disease and in the enhancement of our understanding of the pathophysiologic aspects of autoinflammatory disease. She has a special interest in the application of immunofluorescence in the evaluation and diagnosis of various connective tissue disorders including lupus erythematosus, mixed connective tissue disease, and dermatomyositis along with distinctive vascular syndromes, including Degos disease, cocaine induced retiform purpura, and antiphospholipid antibody syndrome. She has developed the anti-endothelial cell antibody assay to assess for circulating anti-endothelial cell antibodies which are characteristically present in certain diseases such as scleroderma, dermatomyositis, and antiphospholipid antibody syndrome. She has also applied her experience in the field to evaluate neurologic disease, using information gleaned from the immunofluorescent biopsy to predict underlying mechanisms leading to neurologic disease, most notably small fiber neuropathy. Dr. Magro has also applied her expertise in the field of cutaneous immunofluorescence in evaluation of autoinflammatory conditions of the lung, publishing the only comprehensive paper and book chapter devoted to the role of direct and indirect immunofluorescence in the assessment of medical lung disease. Reprints of her work in the field of immunofluorescence are available on request. Please see bibliography below.
Selected Bibliography in the Field of Immunofluroescence:
Magro CM, Roberts-Barnes J, Crowson AN. Direct immunofluorescence testing in the diagnosis of immunobullous disease, collagen vascular disease, and vascular injury syndromes. Dermatol Clin. 2012 Oct;30(4):763-98.
Camp BJ, Magro CM. Cutaneous macroglobulinosis: a case series. J Cutan Pathol. 2012 Oct;39(10):962-70
Magro CM, Rossi A, Poe J, Manhas-Bhutani S, Sadick N. The role of inflammation and immunity in the pathogenesis of androgenetic alopecia. J Drugs Dermatol. 2011 Dec;10(12):1404-11.
Magro CM, Wu R. Anti-laminin 5 pemphigoid: a case report of a benign cutaneous confined non-cicatricial variant. Int J Dermatol. 2012 Jan;51(1):79-85.
Swaminath A, Magro CM, Dwyer E. Refractory urticarial vasculitis as a complication of ulcerative colitis successfully treated with rituximab. J Clin Rheumatol. 2011 Aug;17(5):281-3.
Magro CM, Poe JC, Kim C, Shapiro L, Nuovo G, Crow MK, Crow YJ. Degos disease: a C5b-9/interferon-α-mediated endotheliopathy syndrome. Am J Clin Pathol. 2011 Apr;135(4):599-610.
Rossi A, Reszko A, Leach J, Magro CM. Combined bullous pemphigoid and pemphigus vulgaris in an 18-year-old female. J Cutan Pathol. 2010 Sep;37(9):991-6. Epub 2009 Jul 14.
Magro CM, Dyrsen ME. The use of C3d and C4d immunohistochemistry on formalin-fixed tissue as a diagnostic adjunct in the assessment of inflammatory skin disease. J Am Acad Dermatol. 2008 Nov;59(5):822-33
Magro CM, Schaefer JT, Waldman J, Knight D, Seilstad K, Hearne D. Terbinafine-induced dermatomyositis: a case report and literature review of drug-induced dermatomyositis. J Cutan Pathol. 2008 Jan;35(1):74-81
Magro CM, Ross P, Marsh CB, Allen JN, Liff D, Knight DA, Waldman WJ, Cowden DJ. The role of anti-endothelial cell antibody-mediated microvascular injury in the evolution of pulmonary fibrosis in the setting of collagen vascular disease. Am J Clin Pathol. 2007 Feb;127(2):237-47.
Vasil KE, Magro CM. Cutaneous vascular deposition of C5b-9 and its role as a diagnostic adjunct in the setting of diabetes mellitus and porphyria cutanea tarda. J Am Acad Dermatol. 2007 Jan;56(1):96-104.
Patterson CC, Ross P Jr, Pope-Harman AL, Knight DA, Magro CM. Alpha-1 anti-trypsin deficiency and Henoch-Schönlein purpura associated with anti-neutrophil cytoplasmic and anti-endothelial cell antibodies of immunoglobulin-A isotype. J Cutan Pathol. 2005 Apr;32(4):300-6.
Wusirika R, Ferri C, Marin M, Knight DA, Waldman WJ, Ross P Jr, Magro CM. The assessment of anti-endothelial cell antibodies in scleroderma-associated pulmonary fibrosis. A study of indirect immunofluorescent and western blot analysis in 49 patients with scleroderma. Am J Clin Pathol. 2003 Oct;120(4):596-606.
Magro CM, Pope Harman A, Klinger D, Orosz C, Adams P, Waldman J, Knight D, Kelsey M, Ross P Jr. Use of C4d as a diagnostic adjunct in lung allograft biopsies. Am J Transplant. 2003 Sep;3(9):1143-54
Magro CM, Allen J, Pope-Harman A, Waldman WJ, Moh P, Rothrauff S, Ross P Jr. The role of microvascular injury in the evolution of idiopathic pulmonary fibrosis. Am J Clin Pathol. 2003 Apr;119(4):556-67
Crowson AN, Mihm MC Jr, Magro CM. Cutaneous vasculitis: a review. J Cutan Pathol. 2003 Mar;30(3):161-73. Review.
Crowson AN, Magro CM, Usmani A, McNutt NS. Immunoglobulin A-associated lymphocytic vasculopathy: a clinicopathologic study of eight patients. J Cutan Pathol. 2002 Nov;29(10):596-601
Crowson AN, Magro C. The cutaneous pathology of lupus erythematosus: a review. J Cutan Pathol. 2001 Jan;28(1):1-23. Review.
Magro CM, Morrison C, Pope-Harman A, Rothrauff SK, Ross P Jr. Direct and indirect immunofluorescence as a diagnostic adjunct in the interpretation of nonneoplastic medical lung disease. Am J Clin Pathol. 2003 Feb;119(2):279-89.
Crowson AN, Magro CM. The role of microvascular injury in the pathogenesis of cutaneous lesions of dermatomyositis. Hum Pathol. 1996 Jan;27(1):15-9.
Crowson AN, Magro CM. Diltiazem and subacute cutaneous lupus erythematosus-like lesions. N Engl J Med. 1995 Nov 23;333(21):1429.
Crowson AN, Magro CM. Deposition of membrane attack complex in cutaneous lesions of lupus erythematosus. J Am Acad Dermatol. 1994 Sep;31(3 Pt 1):515-6.
Magro CM, Crowson AN, Harrist TJ. The use of antibody to C5b-9 in the subclassification of lupus erythematosus. Br J Dermatol. 1996 May;134(5):855-62.

